Nitrate Reduction Test – Principle, Procedure, Uses and Interpretation
Anaerobic metabolism requires an electron acceptor other than atmospheric oxygen (O2). Many gram-negative bacteria use nitrate as the final electron acceptor.
Nitrate reduction test is a test that determines the production of an enzyme called nitrate reductase, which results in the reduction of nitrate (NO3).
Bacterial species may be differentiated on the basis of their ability to reduce nitrate to nitrite or nitrogenous gases.
Objectives
- To determine the ability of an organism to reduce nitrate to nitrite.
- Identify the different ways that nitrate can be reduced by bacteria
Principle
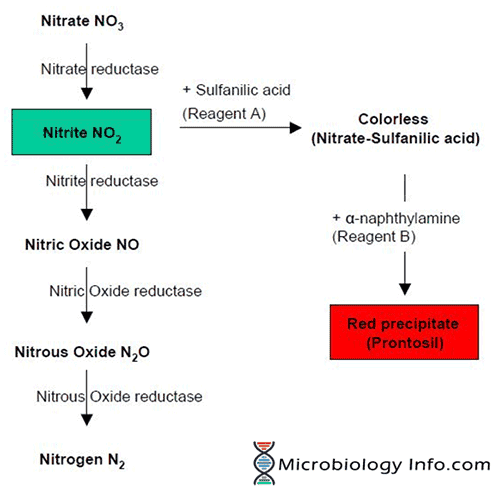
A heavy inoculum of test organism is incubated in a broth containing nitrate. The organisms capable of producing the nitrate reductase enzyme reduce the nitrate, present in the broth, to nitrite which may then be further reduced to nitric oxide, nitrous oxide, or nitrogen.
The nitrate reduction test is based on the detection of nitrite and its ability to form a red compound when it reacts with sulfanilic acid to form a complex (nitrite-sulfanilic acid) which then reacts with a α-naphthylamine to give a red precipitate (prontosil), which is a water-soluble azo dye.
However, only when nitrate is present in the medium, red color will be produced. If there’s no red color in the medium after you’ve added sulfanilic acid and α-naphthylamine means only that nitrite is not present in the medium.
There is two explanations for this observation.
- The nitrate may not have been reduced; the strain is nitrate-negative.
- The nitrate may have been reduced to nitrite which has then been completely reduced to nitric oxide, nitrous oxide, or nitrogen which will not react with the reagents that react with nitrite; the strain is nitrate-positive.
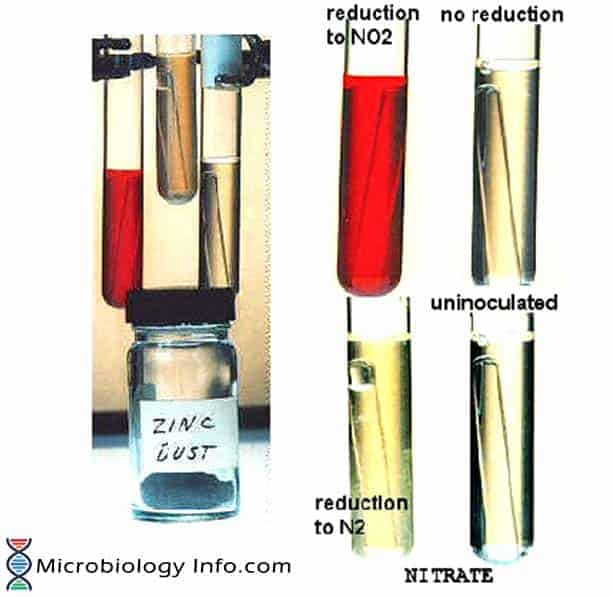
Thus, when nitrite is not detected, it is necessary to test if the organism has reduced nitrate beyond nitrite. This can be done indirectly by adding small amount of Zinc powder to the culture. Zinc powder catalyses the reduction of nitrate to nitrite. The development of the red color on addition of Zinc indicates that nitrate was not reduced by the organism which suggests that the test organism is not capable of reducing nitrate. If no color change occurs after the addition of zinc, this indicates that the organism reduced nitrate to one of the other nitrogen compounds and thus is a nitrate reducer.
Note: A Durham tube is placed in the nitrogen broth in order to detect deterioration of the broth before inoculation, as evidenced by gas formation in the tube and to identify denitrification by organisms that produce gas by alternate pathways.
Media:
Nitrate Broth
Peptone 5 g/l, Meat extract 3 g/l, Potassium nitrate 1 g/l.
Final pH 7.0 ± 0.2 at 25°C
Method
Determination of nitrate reduction to nitrite is a two step process. First, the reduction of nitrate to nitrite is determined by the addition of Nitrate Reagents A and B, then if necessary, the reduction of nitrate beyond nitrite is determined by the addition of Nitrate Reagent C (zinc dust).
- Inoculate the nitrate broths with bacterial suspension.
- Incubate the tubes at the optimal temperature 30°C or 37°C for 24 hours.
- After incubation look for N2 gas first before adding reagents.
- Add 6-8 drops of nitrite reagent A and add the 6-8 drops of nitrite reagent B.
- Observe for the reaction (color development) within a minute or less.
- If no color develops add zinc powder.
- Observe for at least 3 minutes for a red color to develop after addition of zinc.
Expected Results
- Positive Test:
– Development of a cherry red coloration on addition of reagent A and B
– Absence of a red color development on adding Zn powder
- Negative Test:
– A development of red color on addition of Zn powder

Uses
- All members of the Enterobacteriaceae family reduce nitrate, but some members further metabolize nitrite to other compounds. It is thus used to differentiate members of Enterobacteriaceae that produce enzyme nitrate reductase from Gram negative bacteria that do not produce the enzyme nitrate reductase.
- The reduction of nitrate may be coupled to anaerobic respiration in some species.
- It is used in differentiating Mycobacterium
- Identifying species of Neisseriaand separating them from Moraxella and Kingella The nitrate reduction test is a critical test for differentiating between N. gonorrhoeae and K. denitrificans, particularly when strains of K. denitrificans appear to be gram-negative diplococci in stained smears.
- Facilitating species identification of Corynebacterium
Limitations
- The nitrate reduction test may be used as an aid in the identification of bacteria. Additional biochemical testing using pure culture is recommended for complete identification.
- Due to the possible presence of nitrite in the culture media, a low nitrite media such as Nitrate Agar or Nitrate Broth should be used for the nitrate reduction test.
- A negative zinc reduction (no color change) test, in combination with a negative nitrite reaction, is presumptive indication that the nitrate was reduced beyond the nitrite stage. Although a very common end product of nitrite reduction is nitrogen gas, other end products may be formed. Additional testing may be required to determine the final end products of the reaction.
- To avoid false-negative nitrite reduction reactions, negative nitrite reactions must be verified by the addition of zinc dust to the medium.
- Excess zinc dust has been reported to cause false-positive nitrite reduction reactions due to complete reduction of previously unreduced nitrate to ammonia.
References
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- B.D. Skerman, A guide to the identification of the genera of bacteria, The Williams & Wilkins Co., Baltimore, MD, p.218 – 220 (1967)
- https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/Datasheet/1/73426dat.pdf
- www.biologypractical.com/nitrate-reduction-test-objective-principle-procedure-and-result/
- delrio.dcccd.edu/jreynolds/microbiology/2420/files/nitrate.pdf
- https://microbenotes.com/nitrate-reduction-test-objectives-principle-procedure-and-results/
- www.austincc.edu/microbugz/nitrate_reduction.php
- www.asmscience.org/content/education/protocol/protocol.3660
- https://catalog.hardydiagnostics.com/cp_prod/content/hugo/nitratereagent.htm
Similar Posts:
- Selenite F Broth- Composition, Principle, Uses, Preparation and Result Interpretation
- Oxidase Test- Principle, Uses, Procedure, Types, Result Interpretation, Examples and Limitations
- Phenol Red Fermentation Test – Principle, Procedure, Uses and Interpretation
- List of culture media used in microbiology with their uses




